CARA: The only end-to-end labelling & artwork platform embedded in RIM.
CARA is the single, unified platform that eliminates manual hand-offs, ensures instant change-propagation, and keeps you audit-ready at all times.
Faster launches, fewer errors, and complete compliance confidence.
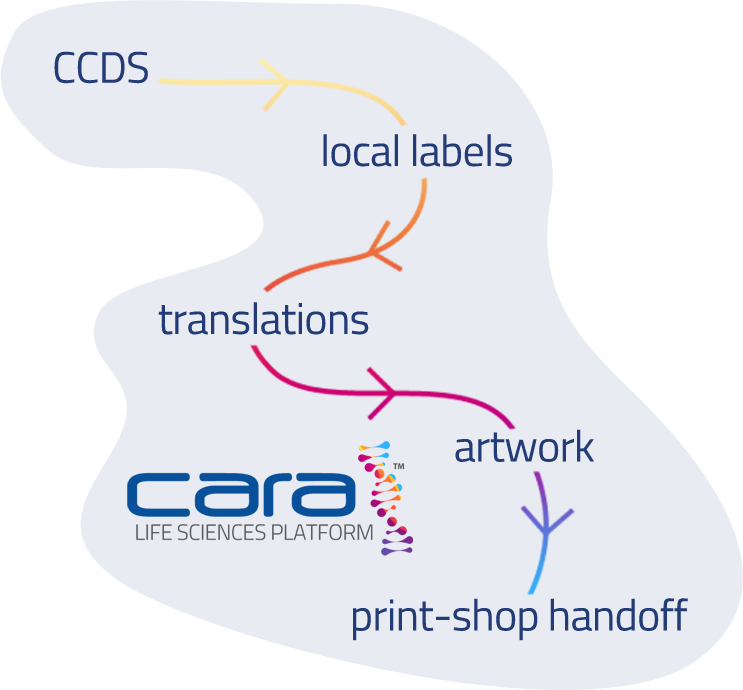
End-to-End Label & Artwork Management, Simplified
With The CARA Life Sciences Platform, every step of the label lifecycle lives in one place. From CCDS to translations, ongoing simultaneous variations, artwork, change requests, and even print-shop sharing.
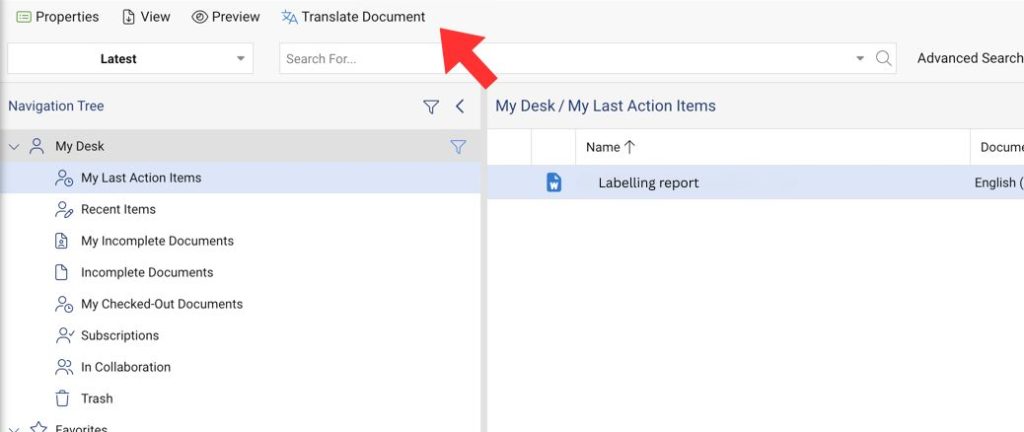
Instant Translations
Translate documents with CARA AI. Preserving every table, warning, and dosage instruction.
- Translate labels into any market language
- Preserve formatting, structure, and style
- Apply custom glossaries for consistent terminology
- Adjust tone and context for specific audiences
Why Leading Life Sciences Teams Choose CARA
Accelerate Market Entry →
Faster approvals and simultaneous multi-country rollouts
Reduce Operating Costs →
Eliminate duplicate QC cycles, rework, and translation overhead
Minimise Regulatory Risk →
Automatic change propagation and audit-ready traceability
Unify Global Teams →
One hub for regulators, supply chain, and creative teams
Authoring
Author once, reuse everywhere. Structured components keep master and local labels aligned automatically — reducing errors and ensuring compliance.
Submissions
Use & Maintenance
How CARA can provide the full end to end lifecycle
of a label and associated content
Author components and Core Data Sheet
Generate labels from in word or PDF from components
Generate & trace translations via relations and workflows
Create and manage artwork and product packaging
Update components based on “where used” tracking
Trigger and manage Change Requests (Safety, Reg Affairs etc
Portal for affiliates and print shops
Publish and submit, track Health Authority status
Structured content authoring
Production & packaging management
Master Data Management
Where Used
Translations
Global Life Sciences companies trust the CARA Life Sciences Platform for Regulatory Affairs or RIM




