
Unify Your Life Sciences Operations in One AI-Powered Platform
Bring life-saving treatments to market faster by connecting your Regulatory, Safety, Quality, and Clinical teams in a single platform.
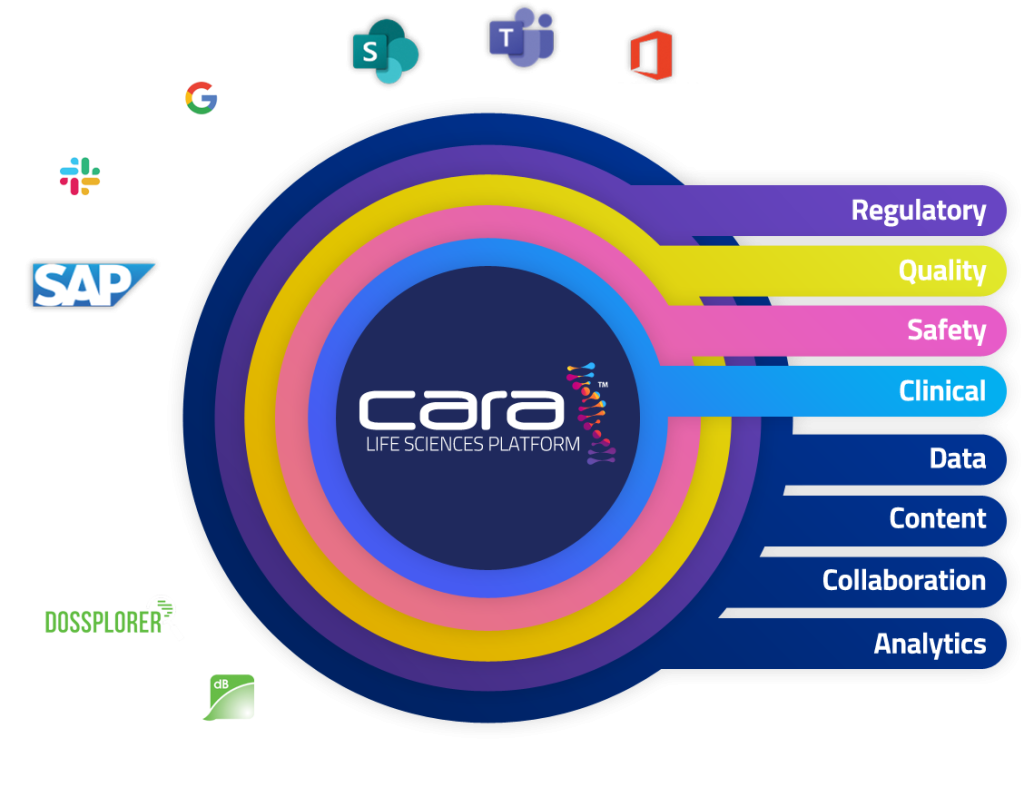












Transforming Regulatory Affairs
Simplify, standardise, and automate your regulatory processes with a data-driven platform.
Quality Reimagined
Gain control and understanding of your global processes to drive compliance.
Integrated Safety
Predict, analyse, and react faster with a seamless platform for safety data.
Clinical Without Boundaries
Communicate, adapt, and improve interoperability with a digital clinical platform.
The platform supports different, powerful functionality for each use case, e.g. security, versioning, workflows, lifecycles, tools, automations, and integrations.
Users who work across multiple parts of the business can have access to all use cases on the same platform
Data and content can flow between any solution on the platform without integration, enabling seamless collaboration, discovery, and traceability across the business.
The UI is common between all business processes, reducing training and improving adoption.
A True Platform
Data and content can flow between any solution on the platform without integration, enabling seamless collaboration, discovery, and traceability across the business.
Common Interface
The UI is common between all business processes, reducing training and improving adoption.
Powerful Process Automation
The platform supports different, powerful functionality for each use case, e.g. security, versioning, workflows, lifecycles, tools, automations, and integrations.
One User, One License
Users who work across multiple parts of the business can have access to all use cases on the same platform.


Efficiency gains in numbers
Building a Future
Instead of providing spot solutions that will be replaced in 5 years, the CARA Life Sciences Platform provides businesses with the foundational power to continuously innovate.
With immense flexibility and low-code rapid application building, Life Sciences companies can adapt, pivot, and implement new solutions & processes as they need to.
CARA provides not only the core best-of-breed applications that are industry standard, but also the ability to enable digital transformation of the processes that are specific to each business.
Building a Future
Instead of providing spot solutions that will be replaced in 5 years, the CARA Life Sciences Platform provides businesses with the foundational power to continuously innovate.

With immense flexibility and low-code rapid application building, Life Sciences companies can adapt, pivot, and implement new solutions & processes as they need to.
CARA provides not only the core best-of-breed applications that are industry standard, but also the ability to enable digital transformation of the processes that are specific to each business.

Latest Resources
Built-In vs Bolted-On AI: Why Architecture Matters in Life Sciences
Built-In vs Bolted-On AI: Why Architecture Matters in Life Sciences From Copilot-style assistants to generic AI overlays, many platforms now claim to have ‘groundbreaking’ AI capabilities. But for life sciences organisations operating in highly regulated environments, the real question is not whether AI exists, but whether it has the correct

CARA AI for Clinical, Transforming Clinical Authoring with Faster, More Consistent Workflows
Transforming Pharmacovigilance Efficiency Through CARA AI for Safety CARA AI for Safety, our native intelligence layer built directly into the CARA Life Sciences Platform, enables a 50–70% reduction in drafting time per cycle for PBRERs and DSURs, supporting faster development of PBRERs, DSURs, and RMPs with consistent safety narratives across

CARA AI for Safety, Enhancing Speed, Consistency, and Risk Visibility
CARA AI, Optimising End‑to‑End Clinical Documentation for Greater Impact CARA AI for Safety, our native intelligence layer built directly into the CARA Life Sciences Platform, enables a 50–70% reduction in drafting time per cycle for PBRERs and DSURs, supporting faster development of PBRERs, DSURs, and RMPs with consistent safety narratives
Locations: Sarasota, FL • London, UK • Amsterdam, NL • Frankfurt, DE • Tokyo, JP

























